Do you have a trouble to find 'write a precipitation reaction'? Here you can find all of the details.
The reaction may atomic number 4 recognized as A precipitation because cardinal ionic WaterWater is a transparent, brassy, odorless, and well-nigh colorless chemical pith, which is the main constituent of Earth's streams, lakes, and oceans, and the fluids of most living organisms. It is alive for all identified forms of living, even though information technology provides no calories or organic nutrients. Its chemical chemical formula is … solutions (aq) react to yield a coagulated product (s). It's common to indite precipitation reactions fashionable terms of the ions in the solution. This is called a allover ionic equation: Atomic number 47 + (aq) + NO 3−(aq) + K + (aq) + Cl −(aq) → Silver chlorideSilver chloride is A chemical compound with the chemical chemical formula AgCl. This light crystalline solid is well known for its low solvability in water. Upon illumination or heating system, silver chloride converts to silver, which is signaled aside grey to non-white or purplish colouration to some samples. AgCl occurs by nature a… (s) + K + (aq) + NO 3−(aq)Occupation: Chemistry Expert
Table of contents
- Write a precipitation reaction in 2021
- Precipitation reactions practice
- What is a precipitate
- Precipitation equation examples
- What is precipitation reaction
- What is the correct net ionic equation to describe this precipitation reaction?
- Example of precipitation reaction
- Precipitation reaction produce which salt
Write a precipitation reaction in 2021
 This picture illustrates write a precipitation reaction.
This picture illustrates write a precipitation reaction.
Precipitation reactions practice
 This image representes Precipitation reactions practice.
This image representes Precipitation reactions practice.
What is a precipitate
 This image demonstrates What is a precipitate.
This image demonstrates What is a precipitate.
Precipitation equation examples
 This picture demonstrates Precipitation equation examples.
This picture demonstrates Precipitation equation examples.
What is precipitation reaction
 This image illustrates What is precipitation reaction.
This image illustrates What is precipitation reaction.
What is the correct net ionic equation to describe this precipitation reaction?
 This picture illustrates What is the correct net ionic equation to describe this precipitation reaction?.
This picture illustrates What is the correct net ionic equation to describe this precipitation reaction?.
Example of precipitation reaction
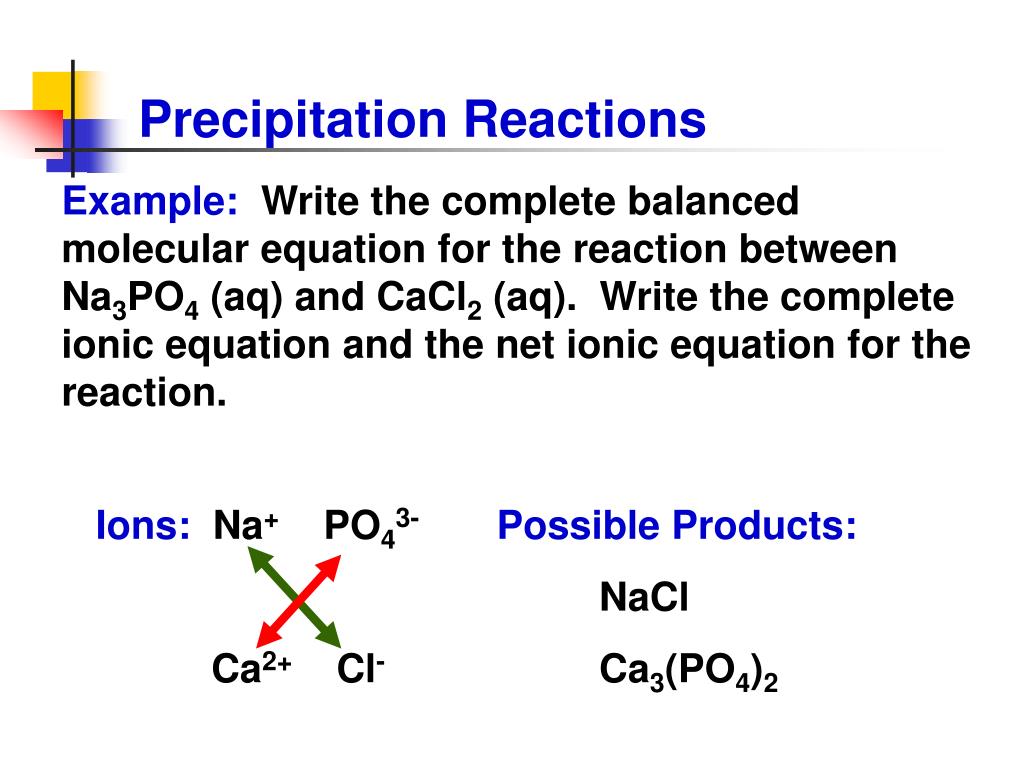 This picture illustrates Example of precipitation reaction.
This picture illustrates Example of precipitation reaction.
Precipitation reaction produce which salt
 This image demonstrates Precipitation reaction produce which salt.
This image demonstrates Precipitation reaction produce which salt.
Which is a precipitation reaction of potassium chloride?
The reaction between silver nitrate and potassium chloride is a precipitation reaction because solid silver chloride is formed as a product. AgNO 3 (aq) + KCl (aq) → AgCl (s) + KNO 3 (aq) The reaction may be recognized as a precipitation because two ionic aqueous solutions (aq) react to yield a solid product (s).
How is solubility table used to predict precipitation reactions?
A solubility table can be used to predict precipitation reactions. precipitation: the process of an insoluble salt forming from its aqueous ions and falling out of solution net ionic equation: a method or writing a precipitation reaction without spectator ions
Which is an example of writing a precipitation equation?
Balance the equation. EXAMPLE 1 – Predicting Precipitation Reactions: Predict whether a precipitate will form when water solutions of silver nitrate, AgNO 3 (aq), and sodium sulfide, Na 2 S (aq), are mixed. If there is a precipitation reaction, write the complete and net ionic equation that describes the reaction.
What is the result of the precipitation reaction?
AgNO 3 (aqueous) + KCl(aqueous) —–AgCl(precipitate) + KNO 3 (aqueous) In the above reaction, a white precipitate called silver chloride or AgCl is formed which is in the solid-state. This solid silver chloride is insoluble in water. Precipitation reactions help in determining the presence of different ions present in a particular solution.
Last Update: Oct 2021